INTRODUCTION
Corona virus disease 19 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2. It has affected almost 200 million people and caused more than 4 million deaths worldwide [1]. Due to its high contagiousness and lack of effective treatment, the COVID-19 vaccine had to be developed promptly. The Republic of Korea started vaccination in February 2021 with the Pfizer-BioNTech mRNA-based (BNT162b2) and Oxford-AstraZeneca non-replicating viral-vectored (ChAdOx1 nCoV-19) vaccines [2]. However, unusual thrombotic events and thrombocytopenia developed in individuals who obtained the ChAdOx1 nCoV-19 vaccine. In March 2021, the European Medicines Agency reported a possible link between the vaccine and very rare cases of unusual blood clots with low blood platelet levels [3]. Many thrombotic events have been reported, but most cases are related to cerebral venous sinus thrombosis, and cases of arterial thrombosis are rare. In South Korea, there was only one fatal case of thrombosis with thrombocytopenia, which was related to cerebral venous sinus thrombosis. The present case illustrates a fatal case of cerebral arterial thrombosis shortly after COVID-19 vaccination. The association between this case and COVID-19 vaccination has not yet been proven, but its possibility should never be neglected.
CASE REPORT
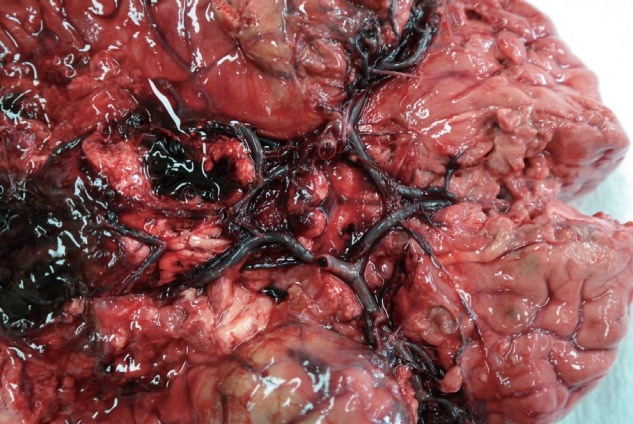
The deceased patient was a 63-year-old woman. After the initial COVID-19 vaccination with ChAdOx1 nCoV-19, the patient presented with symptoms of nausea, fatigue, and mild headache. These symptoms did not resolve with administration of IV fluid and nonsteroidal antiinflammatory drugs. Thus, the patient was hospitalized 8 days after vaccination. After 2 days, the patient suddenly presented with vomiting, high blood pressure, and fell into a coma. The patient died 23 days after the vaccination. The patient was normal upon external examination. The incision scar for craniotomy was located on the right side of the forehead. the brain was evidently edematous and friable. Subarachnoid hemorrhage was distributed around the brain base, especially around the brain stem where large blood clots remain. The cerebral arteries and internal carotid arteries were enlarged and condensed with thrombi, but neither aneurysms nor atherosclerotic changes were found in this cerebral artery group (Fig. 1). Specimens were collected from the circle of Willis, the posterior cerebral artery, and the internal carotid artery. The heart was normal, but mild atherosclerotic changes in the left anterior descending coronary artery were observed; no pulmonary embolism was observed. Microscopically, the arterial thrombi were firmly attached along the injured endothelial lining (Fig. 2). The thrombi were composed of a tangled mesh of erythrocytes, fibrin, and degenerating leukocytes. Hemorrhagic infarction of the brain stem was also encountered, and the hemorrhage extended towards the subarachnoid space. The cause of death was determined to be brain infarction due to cerebral arterial thrombosis.
DISCUSSION
Ischemic occlusions due to atherosclerotic changes contribute to approximately 85% of casualties in stroke patients. Moreover, intracerebral bleeding caused by ruptured aneurysms, arteriovenous malformation, and head trauma can contribute to stroke [4]. ŌĆśVaccine-induced immune thrombotic thrombocytopenia (VITT)ŌĆÖ is one of the side effects confirmed to be related with COVID-19 vaccination. It is a new clinical syndrome characterized by thrombosis at atypical sites (such as cerebral venous sinus thrombosis, splanchnic vein thrombosis, and arterial thrombosis) combined with thrombocytopenia after vaccination [5]. The American Society of Hematology described all four criteria needed to diagnose this syndrome: 1) symptoms within 42 days after COVID-19 vaccination, 2) any venous or arterial thrombosis, 3) thrombocytopenia (which might be normal in the early stage), and 4) positivity for the PF4 antibody [6]. In case reports of thrombosis after COVID-19 vaccination with ChAdOx1 nCoV-19, patients presented with symptomatic thrombosis at 5 to 21 days after vaccination [7-9]. Sites of thromboses varied; most of the cases included cerebral venous sinus thrombosis, and other cases included occlusion of the middle cerebral artery, internal carotid artery, etc. Symptoms varied from headache to dysphasia, hemiparesis, epileptic seizure, and focal neurologic symptoms (such as aphasia and hemianopsia) [7-9]. Most of the patients presented with mild to moderate thrombocytopenia and were positive for immunoglobulin G (IgG) against the PF4-heparin complex [9]. There was one reported case with a normal platelet count (217,000/╬╝L) and fibrinogen level (2.7 g/L), and highly elevated serum IgG antibodies against PF4 complexes [8]. The American Society of Hematology also describes the possibility of normal platelet count in the early stage of the syndrome [6]. D-dimer levels were elevated (11,220-34,000 ╬╝g/L) in some cases [7]. In this case, the platelet level was within the normal range (346,000/╬╝L), and the medical record of PF4 antibody test was absent. It is known that this case does not meet the diagnostic criteria for VITT, and that clear evidence cannot be provided on how this case is related to vaccination. However, the following three facts are noted in this case: 1) all the symptoms of the deceased occurred immediately after vaccination, 2) the main cause of death was consistent with the known side effects of the vaccine, and 3) the mechanism of thrombus formation in this case goes beyond the general category of pathogenesis known so far. Three primary factors predispose individuals to thrombus formation, the so-called Virchow triad: 1) endothelial injury 2) stasis or turbulence of blood flow, and 3) blood hypercoagulability. Endothelial injury is the dominant influence in that it, by itself, can lead to thrombosis. It is particularly important for thrombus formation occurring in the heart or arterial circulation, where normal flow rates might otherwise hamper clotting by preventing platelet adhesion or diluting coagulation factors. Thus, thrombus formation within the cardiac chambers, and over ulcerated plaques in atherosclerotic arteries or at sites of traumatic or inflammatory vascular injury (vasculitis) is largely due to endothelial injury. Turbulence and stasis clearly contribute to thrombosis in a number of clinical settings, such as aneurysms, myocardial infarctions, mitral valve stenosis, atrial fibrillation, and hyperviscosity syndromes. Hypercoagulability contributes less frequently to thrombotic states and is loosely defined as any alteration in the coagulation pathways that predispose individuals to thrombosis. The cause of hypercoagulability may be through genetic or acquired disorders [10,11]. To track the cause of thrombosis in this case, morphologically identifiable causes, such as atherosclerosis, myocardial infarction, and mitral valve stenosis, were excluded. Those causes are widely known to correspond to dominant causes in thrombogenesis. Arterial thrombi tend to grow in a retrograde direction from the point of attachment, whereas venous thrombi extend in the direction of the blood flow. In this case, the arterial endothelial cells, where selective biopsies were performed, were damaged. Additionally, thrombi were also attached along the endothelial lining of these damaged blood vessels. The authors assessed that these unusual morphological changes were not independent of changes in the blood composition. Unfortunately, the ability to track changes in blood composition in autopsy cases is limited because of changes that occur post-mortem. Due to these limitations, we could not provide solid evidence that this case was related to vaccines. However, we suggest that the criteria for determining vaccine-related side effects should not be limited to VITT. Cases that recognize vaccine-related side effects in Korea tend to be limited to those that meet the VITT criteria [12]. What should not be overlooked in determining whether it is related to the side effects of the vaccine is that the standards are urgently created based on data that are only a little over a year old. If the case is out-of-the-box in the pathogenesis of thrombosis and is related to the known side effects of a specific vaccine, it may not be insufficient data that does not meet the criteria; it can be the basic data for extending and changing incomplete criteria. There is a greater risk of limiting the scope of side effects with a few published categories when the categories of side effects are not clearly established; it is possible to overlook or miss more serious risks that have not yet been revealed and may appear in the future. Nevertheless, the authors know that this case does not fall into some known categories of side effects of vaccination. However, we are presenting this case since at this point, a comprehensive review of various possibilities related to side effects of vaccine is needed to establish a COVID-19 defense system.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print