Rapid progression of large intracranial cerebral artery involvement in a patient with myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis
Article information
Abstract
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a systemic necrotizing vasculitis that predominantly affects small vessels of the body. The two most common ANCAs are myeloperoxidase ANCA and proteinase 3 ANCA. Neurological manifestations are frequent in patients with AAV, including peripheral neuropathy, meningitis, and stroke. AAV-associated ischemic stroke usually affects small vessels supplying the white matter or brainstem. This case report details the presentation and treatment course of a 70-year-old man with rapidly progressive multiple intracranial large artery involvement attributed to myeloperoxidase ANCA-associated vasculitis. Despite treatment with high-dose steroids and a rituximab infusion, the patient developed new speech difficulties and respiratory distress, and brain imaging confirmed new stroke lesions with progressive multiple intracranial large cerebral artery involvement. The patient died from SARS-CoV-2 infection 4 months after the diagnosis. This case emphasized the rare presentation of rapidly progressive large vessel involvement in a patient with myeloperoxidase ANCA-associated vasculitis despite active immunotherapy.
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterized by noninfectious necrotizing vasculitis, specifically distinct from immune complex small vessel vasculitis. The two most common ANCAs are myeloperoxidase ANCA and proteinase 3 ANCA. AAV encompasses conditions such as granulomatosis with polyangiitis, microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis according to clinicopathological characteristics. ANCA not only plays a critical role in disease onset, but its type can influence both prognosis and clinical significance [1]. The prevalence of individual AAV subtypes varies among different populations. Therefore, both genetic and environmental factors significantly influence the immunopathogenesis of AVV. myeloperoxidase ANCA-positive microscopic polyangiitis is the most frequent type of AAV in East Asia [2]. While AAV typically involves small vessels affecting organs, including the kidneys, lungs, and the upper respiratory tract, this chronic vasculitis is also known to elevate the risk of cardiovascular diseases (CVD). In AVV patients, CVD is the primary cause of death, particularly linked to myeloperoxidase ANCA positivity [3]. While the risk of CVD in AAV remains controversial due to different study designs, comparator populations, and case ascertainment strategies, significantly increased incidence of stroke in patients with AAV compared with the general population have been reported [4,5]. Premature atherosclerosis linked to inflammation was suggested as a key factor behind the increased CVD risk in AAV patients [5]. Although the patterns of strokes specific to AAV have not been extensively studied, strokes in AAV patients primarily manifest as ischemic events with lesions predominantly located in the distal penetrating vessel region [6].
In this report, we aim to highlight the unusual presentation of a 70-year-old patient with AAV, demonstrating a rapid progression of large intracranial cerebral arteries, a phenomenon not frequently documented in the literature. This case report was approved by the ethics committee of the Jeju National University Hospital (IRB No. 2023-11-003).
CASE REPORT
A 70-year-old man visited the emergency room due to increased speech difficulty. This speech issue had been present for 20 days, accompanied by pain in the right ear, but had worsened over the last 3 days.
Upon initial examination, the patient’s blood pressure was 149/89 mmHg, pulse 82/min, respiration 20/min, and temperature was 37.2℃. Neurological examination revealed a slight decrease in muscle strength and sensory reduction on the right side of the body, in addition to dysarthria. An initial brain magnetic resonance (MR) image showed multiple small diffusion restriction lesions on the right lateral thalamus and bilateral corona radiata (Fig. 1). Microbleed lesions were observed on the left basis pontine. The patient lacked any risk factors commonly linked to CVDs.
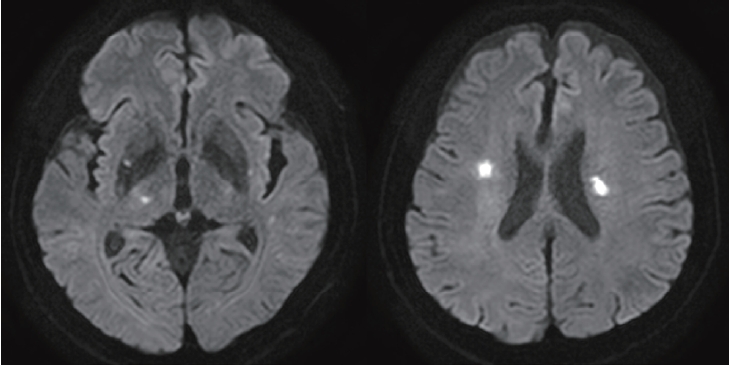
Diffusion-weighted magnetic resonance imaging of the patient showed several areas of restricted diffusion (decreased ADC value) at bilateral corona radiata, right putamen, and right thalamus. ADC: apparent diffusion coefficient.
After admission, the patient presented with an exacerbation of symptoms, characterized by a high fever and increased ear pain. Laboratory tests reveal an evidence of inflammation with a high erythrocyte sedimentation rate (113 mm/h; normal range, <9) and an increased C-reactive protein level (5.64 mg/dL; normal range, <0.30). The presence of microscopic hematuria with dysmorphic red blood cells in the urine analysis suggested possible renal involvement. The serum creatinine levels were 0.81 mg/dL (normal range, 0.80-1.30). Serological tests for syphilis, human immunodeficiency virus, hepatitis C, and hepatitis B were negative. Chest x-ray revealed no abnormalities. No other obvious hematological or biochemical abnormalities were observed. Additionally, cardiovascular assessment via electrocardiogram and echocardiogram showed no abnormalities.
As systemic vasculitis was suspected, the patient underwent further diagnostic evaluation with an internal medicine consultant. Conventional angiography demonstrated multifocal moderate stenosis at bilateral posterior cerebral arteries and segmental stenosis at left distal A2 segment (Fig. 2). The patient showed a high titer for myeloperoxidase ANCA (>135.0 IU/mL; normal, <5.0), and the proteinase 3-ANCA and anti-nuclear antibodies were negative. Computed tomography (CT) of the thorax showed interstitial lung disease (Fig. 3).
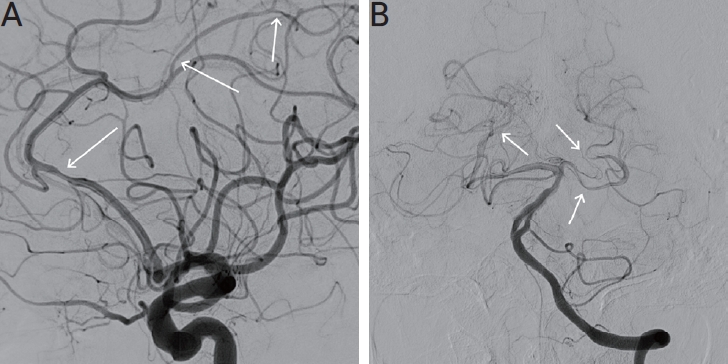
Digital subtraction angiography showed a stenosis of the left anterior cerebral artery (arrows) (A) and multifocal bilateral posterior cerebral arteries (arrows) (B).
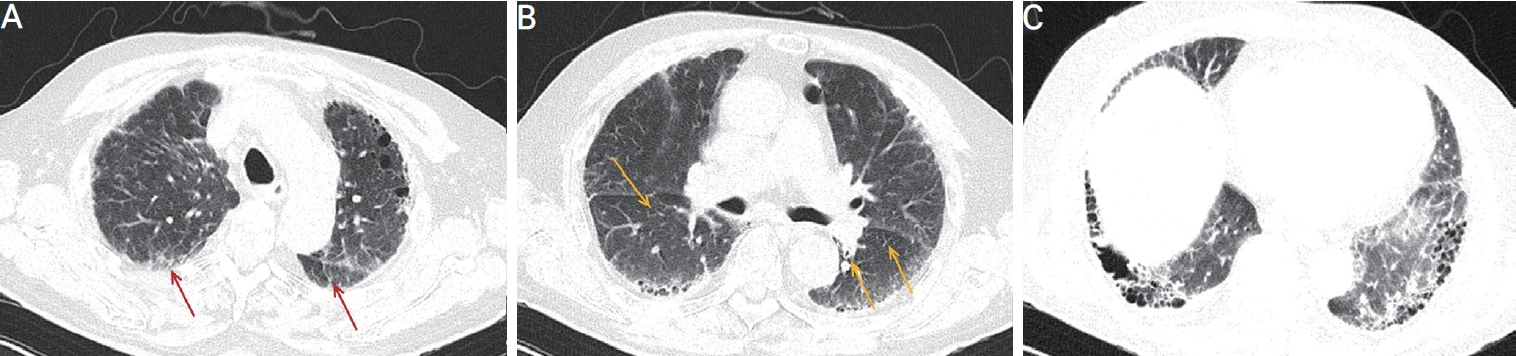
Chest computed tomography showed subpleural reticular densities (red arrows) (A), peribronchial reticular densities and traction bronchiectasis (yellow arrows) (B), and mixed ground-glass opacity and honeycombing appearance in both the lungs with basal predominance (C).
After treatment with a high-dose steroid (methylprednisolone 1,000 mg for 3 days), the patient’s ear pain improved significantly. Subsequently, he was orally administered a methylprednisolone maintenance dose (40 mg twice daily) and an additional 4-week rituximab (375 mg/m2) induction therapy. Despite the fourth rituximab infusion, the patient manifested new speech impairment and respiratory difficulties. Neuroimaging highlighted new ischemic areas in the left subcortical white matter (Fig. 4A) and right external capsule, accompanied by the new appearance of left middle cerebral artery stenosis at the inferior division (Fig. 4B).
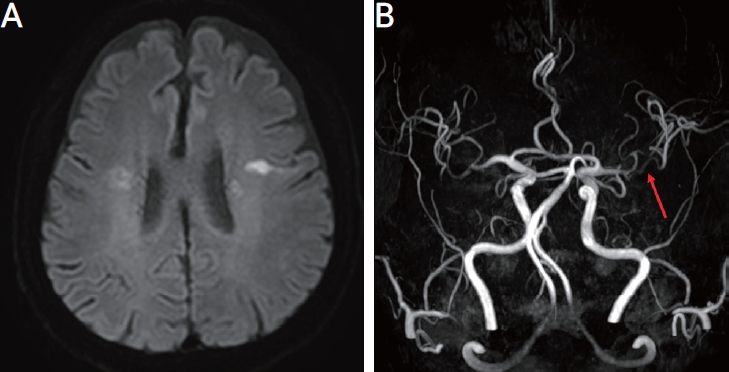
Diffusion-weighted magnetic resonance imaging revealed a new area of subcortical infarction at the white matter of left precentral gyrus (A) and magnetic resonance angiography showed segmental stenosis (red arrow) on the inferior division of left middle cerebral artery (B).
Concurrently, a chest CT identified pneumonia attributed to alveolar hemorrhage. These findings collectively suggest a progression of his MPA. Despite recommencement of steroids and a switch to methotrexate, the patient died due to SARS-CoV-2 infection 4 months after AAV diagnosis.
DISCUSSION
ANCA-associated vasculitis is a representative small-vessel vasculitis, known to affect vessels ranging from arterioles to venules. The primary involvement of small vessels is the hallmark of AAV. Strokes in patients with ANCA-associated vasculitis typically occur due to inflammation, obstruction, or increased permeability of small to medium vessels. Although the frequency of strokes in patients with ANCA-associated vasculitis are unknown, one explanation for large artery involvement is the acceleration of subclinical atherosclerosis due to systemic and localized inflammation and an imbalance of helper T-cells [7]. Mechanically, the development of atherosclerotic lesions is considered an important factor in the occurrence of stroke. In the case of stroke secondary to arteritis, various triggers are known, namely that strokes can occur after an acute infection or after its resolution. Furthermore, an incomplete treatment of a preceding infection or the natural course of the disease can lead to catastrophic outcomes [8].
Determining a diagnosis for cerebral vasculitis can be complex due to the wide range of potential causes, including infections and tumors. Given the patient’s cerebrovascular and renal involvement alongside a high myeloperoxidase ANCA titer and ear pain, the most likely diagnosis was MPA, despite the absence of pathological vessel findings. Furthermore, while a brain biopsy did not confirm a direct link between the stroke and MPA, the presentation of bilateral multiple subcortical infarct patterns suggested that the ischemia of penetrating vessels was likely responsible for the stroke. The intracranial MR angiography suggested that inflammation in the medium and large vessels may have caused progressive multifocal segmental stenosis in this patient. The presence of ANCA reactivity specifies MPA as distinct from other diseases involving medium-size arteritis and the coexistence with pulmonary and nephropathic features, in our case, strengthened the possibility of systemic small-vessel vasculitis.
Clinical evidence shows that rituximab, as used in our patient, can be effective for both induction and maintenance therapy in ANCA-associated vasculitis [9]. Despite this, not all patients respond favorably to this treatment. The prognosis and therapeutic approach for AAV can vary based on the organs involved and disease activity, leading to the development of the five-factor score (FFS) as a prognostic assessment tool for systemic necrotizing vasculitis by the French Vasculitis Study Group [10]. FFS takes into account five key factors: age over 65 years, renal insufficiency, gastrointestinal signs, cardiac insufficiency, and the absence of ear, nose, or throat manifestations. Central nervous system manifestations are not included in the prognosis assessment. However, the involvement of larger intracranial cerebral arteries, as observed in this patient, may suggest a more aggressive form of AVV, which can lead to recurrent strokes.
Such a rapid progression of intracranial large artery involvement, despite active immunosuppressive treatment, poses significant clinical challenges and emphasizes the need for a better understanding of the aggressive form of AVV. These findings warrant several considerations. First, unidentified triggers or co-factors may contribute to progressive large cerebral artery stenotic lesions, causing recurrent strokes in spite of active immunosuppressive treatment. Furthermore, the failure of treatment with high-dose steroids and rituximab in halting the development of new vasculitis lesions suggests that a different therapeutic approach is needed for such unusual cerebrovascular presentations, or that the disease itself has varied subtypes with distinct treatment responses.
Acknowledgements
This work was supported by the 2023 education, research and student guidance grant funded by Jeju National University.
