Pathologic change of an arterialized giant venous aneurysm of a brachiocephalic arteriovenous fistula
Article information
Abstract
Aneurysmal venous dilatation is a frequent complication of arterio venous fistulas (AVFs) created for hemodialysis. Venous aneurysm rupture can lead to lethal hemorrhage. A 49-year-old male patient presented with a giant aneurysmal dilatation of his AVF 10 years after its creation. The patient had complaints of pulsating pain and discomfort due to swelling of the left forearm. We performed an aneurysm resection and revised the overlying dermal lesion through a brachial plexus block. Here, we describe the pathological features of the arterialized venous aneurysm compared to simple venous aneurysms.
INTRODUCTION
Approximately 36% to 39% of patients undergoing hemodialysis are hospitalized for reasons such as catheter-related complications, thrombolytic therapy, and treatment of infection related to vascular access [1]. Although venous aneurysms are rare, they are often difficult complications in patients undergoing hemodialysis. Aneurysms or pseudoaneurysms can develop in approximately 8% of arteriovenous fistulas (AVFs) created for hemodialysis [2]. When a tortuous aneurysmal AVF dilatation develops, venous catheter cannulation can become difficult, and blood flow can be compromised due to thrombus formation within the aneurysm. Additionally, aneurysmal degeneration can cause the breakdown of the overlying skin, thus increasing the risk of bleeding, infection, and deformity of the affected limb [3]. For these reasons, surgical intervention should be considered. Here, we describe the surgical management and pathologic features of a case of arterialized venous aneurysm compared to simple venous aneurysm.
CASE REPORT
A 49-year-old male patient with end-stage renal disease and a history of diabetes mellitus for over 20 years had undergone the creation of a left antecubital brachiocephalic AVF (end-to-side anastomosis of the radial artery and cephalic vein) for hemodialysis access 12 years prior. The patient was referred from the nephrology department to the vascular surgery unit due to a large 10 cm-sized pulsatile mass accompanied by swelling and pain in the left forearm for 3 years. Its progressive growth accelerated over the months prior to the presentation. The venous aneurysm seemed to be non-functional. A new AVF was created on the opposite arm 2 months ago to continue dialysis.
We decided to excise the AVF aneurysm and revise the skin defect under a brachial plexus block. The patient was placed in the supine position with the left arm spread out. There were aneurysmal changes with pulses from the antecubital area to the supraclavicular area along the course of the cephalic vein. The aneurysm measured over 10 cm at its maximal diameter. A separate skin incision was made at the medial portion of the upper arm to control the proximal brachial artery using a vessel loop (Fig. 1A). A longitudinal skin incision along the aneurysm was performed. We then identified the course of the radial artery, the anastomosis site of the fistula, and the proximal portion of the cephalic vein. Each vessel was clamped using vascular clamps following the intravenous infusion of heparin (5,000 IU). We opened the aneurysm and excised it completely. The remaining redundant skin was trimmed and closed with multiple interrupted sutures using tension-free 3-0 nylon. A drainage catheter was placed into the empty space in which the aneurysm was removed. The aneurysm was composed of a thickened wall and filled with organized thrombi (Fig. 1B, asterisks). Postoperatively, the radial pulse was intact, and the edema of the arm had resolved. The drainage catheter was removed on the second postoperative day. The stitches were removed 2 weeks postoperatively. The patient was discharged 9 days postoperatively. He was followed up for 2 years without any complications.

Intra-operative findings. (A) Intra-operative view shows a circumferentially dissected aneurysmal vein with the proximal afferent artery and the root of the aneurysm (asterisk). (B) The excised aneurysmal vein shows significant wall thickening and intravascular thrombi (asterisks).
The histological findings, hematoxylin and eosin (Fig. 2A) and Masson’s trichrome staining (Fig. 2B) showed marked intimal thickening and calcifications in the arterialized vein with extensive collagen depositions in the tunica intima and media revealing mucoid degeneration and loss of the normal smooth muscle.
Histological findings of arterialized venous aneurysmal wall. The media shows haphazard disorganization, loss of smooth muscle cells with mucoid degeneration (H&E, ×200). The mucoid deposition in the intima and media are accompanied by destruction of the elastic fibers (A). Masson’s trichrome stain reveals extensive collagen depositions in the tunica intima and media. Multi-focal calcifications (arrows) are also noted (Masson’s trichrome stain, ×40) showing severe infiltration of collagenous and fibrous tissues. There is a significant loss of the normal arrangement of medial smooth muscle cells with diffuse and focal intimal hypertrophy and the accumulation of collagen fibers in both the intima and media with the replacement of smooth muscle cells by collagen fibers (B). H&E: hematoxylin and eosin.
DISCUSSION
The most frequent late complication of AVFs for hemodialysis is aneurysmal dilatation, which can be either true, containing all layers of the venous wall, or false, lined by fibrous tissue and thrombi. Aneurysmal dilation of the vein may occur because of repeating punctures that can weaken the venous wall in some patients. Long-standing high turbulent blood flow rates through an AVF result in shear forces damaging the elastic fibers of the internal elastic lamina, often resulting in a progressive increase in size [4,5]. Sometimes, proximal or outflow vein stenosis accelerates the process by raising the pressure into the AVF.
Patients with AVF aneurysms have been well documented as experiencing symptoms such as pain, swelling, thrombi, and ruptures, which lead to surgical excision. While spontaneous rupture of true AVF aneurysms is very rare, it may occur if the aneurysmal segment is directly cannulated [6]. Untreated aneurysms can lead to short-term complications, such as symptoms resulting from local compression, emboli, endocarditis, and rupture, or long-term complications, such as dilatation, venous hypertension, or distal ischemia [6].
There have been several reports regarding the management of AVF aneurysms, including surgical ligation and resection, ultrasonography-guided compression, endovascular stent implantation, perivascular metal meshes, or thrombin injections [7]. Endovascular interventions have increased in recent years. However, this method has some disadvantages, such as higher costs or infection due to foreign bodies. Surgical repair is an effective method for the elimination of the aneurysm, and the risk of bleeding during surgery is less compared to other methods [8].
The published evidence that aneurysmal dilatation occurs in the arterialized vein is limited. AVF aneurysm is a result of extensive calcification as well as the significant increase of myofibroblasts, which represent the major source of extracellular collagen deposition within the venous wall [9]. The arterialized vein in AVF sites is presumed to have the same characteristics as arteries. Vascular calcification, which usually occurs within arteries, has happened in the intimal layer and medial wall, or tunica media, of arteries within atherosclerotic plaques. Wail et al. [10] reported that the cephalic veins, after AVF construction, presented with thickening of the wall due to intimal hyperplasia and replacement by collagenous, fibrous tissue. Histological examination of resected fistula aneurysms demonstrates extensive infiltration of collagen with thickening and altered architecture of the vessel walls. We compared it with the histopathologic features of a simple cephalic venous aneurysm (Fig. 3). This is the tissue of a 68-year-old male patient who visited the hospital with a cystic lesion on his arm, and computed tomography angiography confirmed it as a venous aneurysm and underwent a resection. Any atherosclerotic changes such as calcification, cholesterol clefts, and irregular and haphazard proliferation of smooth muscle cells, which are characteristics of arterialized veins, were not found.

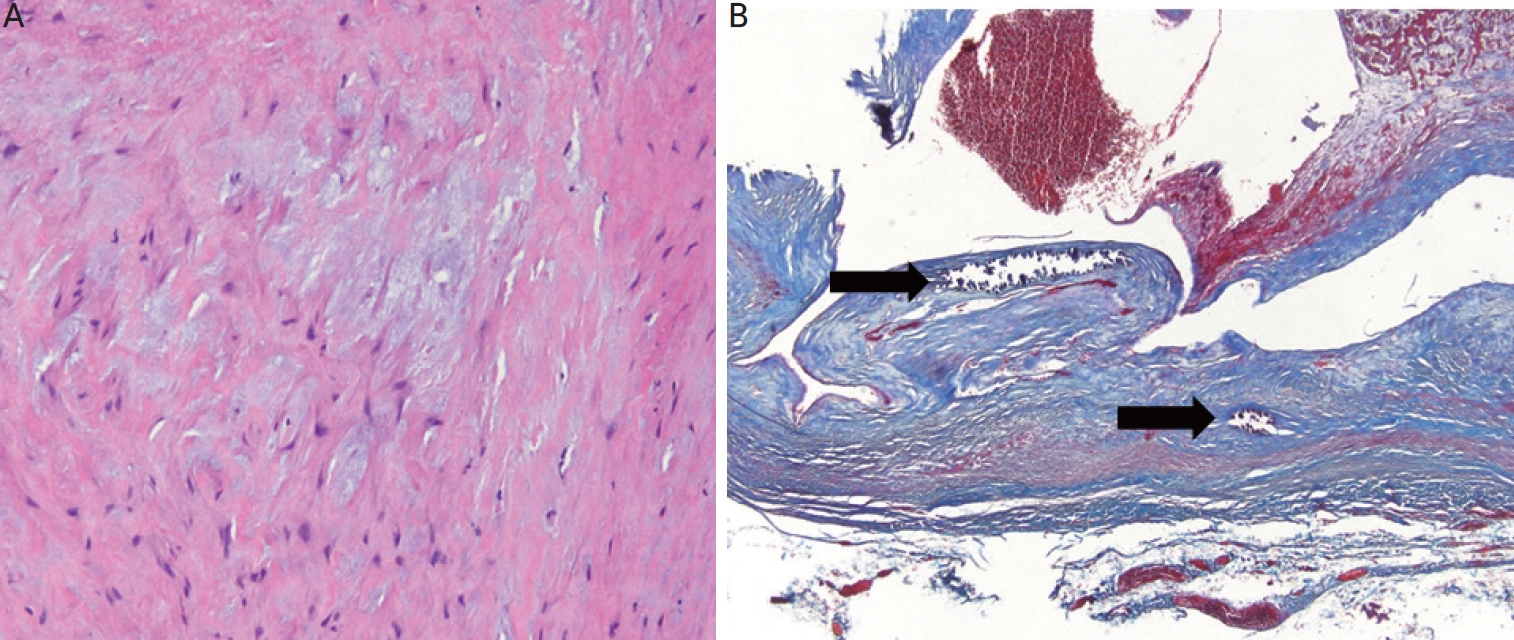
Histomorphologic findings of simple venous aneurysmal wall. At a high magnification, (A) thick-walled vascular tissue with medial hypertrophy and myxoid degeneration (H&E, ×200). (B) Collagen fiber deposition in the thickened vascular wall (Masson’s trichrome stain, ×40), but there are no observed atherosclerotic changes such as calcifications, cholesterol clefts, or haphazard proliferation of smooth muscle cells. H&E: hematoxylin and eosin.
This study is an attempt to identify the pathologic features of giant AVF aneurysms compared with simple venous aneurysms and to describe the surgical result of management. We suggest that non-functioning giant AVF aneurysms should be excised, and the operation is not complicated.
Acknowledgements
This work was supported by the 2023 education, research and student guidance grant funded by Jeju National University.
