Insight into the transcriptome of the small intestine in pups born from corticosterone-treated rats
Article information
The brain-gut axis plays a critical role in modulating gastrointestinal (GI) function through the autonomic nervous system. Stress leads to pathological conditions in the GI tract, which affects the gut microbiome composition, secretion, intestinal permeability. Corticosterone is a steroid hormone produced in the adrenal cortex and is associated with stress. Previous studies showed that corticosterone plays a vital role in regulating various aspects of GI function. This hormone can affect permeability, the microbiome, and inflammation, and its dysregulation can lead to the development of GI disorder [1,2]. Corticosterone has been administered to animal models to investigate the impact of chronic stress, and cortisol levels are elevated in pups born from pregnant rats treated with corticosterone [3]. In this study, we employed 2-week-old pups born from rats administered with corticosterone to investigate the transcriptome of smooth muscle isolated from the small intestine. Corticosterone (20 mg/kg) was subcutaneously administered to pregnant rats (8-9 weeks old female Sprague-Dawley rats) daily for 21 days, beginning from the confirmation of fertilization until delivery, to elevate the bloodstream levels of cortisol. As controls, pregnant rats were treated daily with saline (0.9% NaCl) rather than with corticosterone. All animal experiments and procedures were approved by the Animal Care and Use Committee of Jeju National University (No. 2023-0026). The smooth muscle layers were isolated by physically stripping them from the intact mucosa of the small intestine and then evaluated using RNA-sequencing. The isolated smooth muscle tissues were preserved in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to polyA-enriched RNA-sequencing to quantify the mRNA expression.
The kallisto tool was used to summarize the genome-wide gene expression profiles from the RNA-sequencing data based on the Ensembl genome annotation (mRatBN7.2). The edgeR package was used to identify differentially expressed genes in pups born from the controls and from corticosterone-treated rats. Genes with a false discovery rate <10% and fold-change >1.5 were considered as differentially expressed. The gene names are described in Supplementary Table 1.
We observed differential expression of various genes associated with calcium signaling and ion channels in pups born to corticosterone-treated rats compared with that in control pups. Among ion channel genes, the expression of Scn1a, Grin3a, and Htr3b was decreased in pups born to corticosterone-treated rats compared to that in control pups (Fig. 1). SCN1A, a voltage-gated sodium channel, is primarily expressed in the brain but can also be found in the GI tract, where it contributes to modulating visceral pain. GRIN3A encodes the glutamate ionotropic receptor N-methyl-D-aspartate-type subunit 3A, whereas Htr3b encodes the serotonin receptor 3B, a subtype of the serotonin receptor. Enterochromaffin cells are responsible for the synthesis of most serotonin in the GI tract, where they play regulatory roles in motility, secretion, and visceral sensitivity.
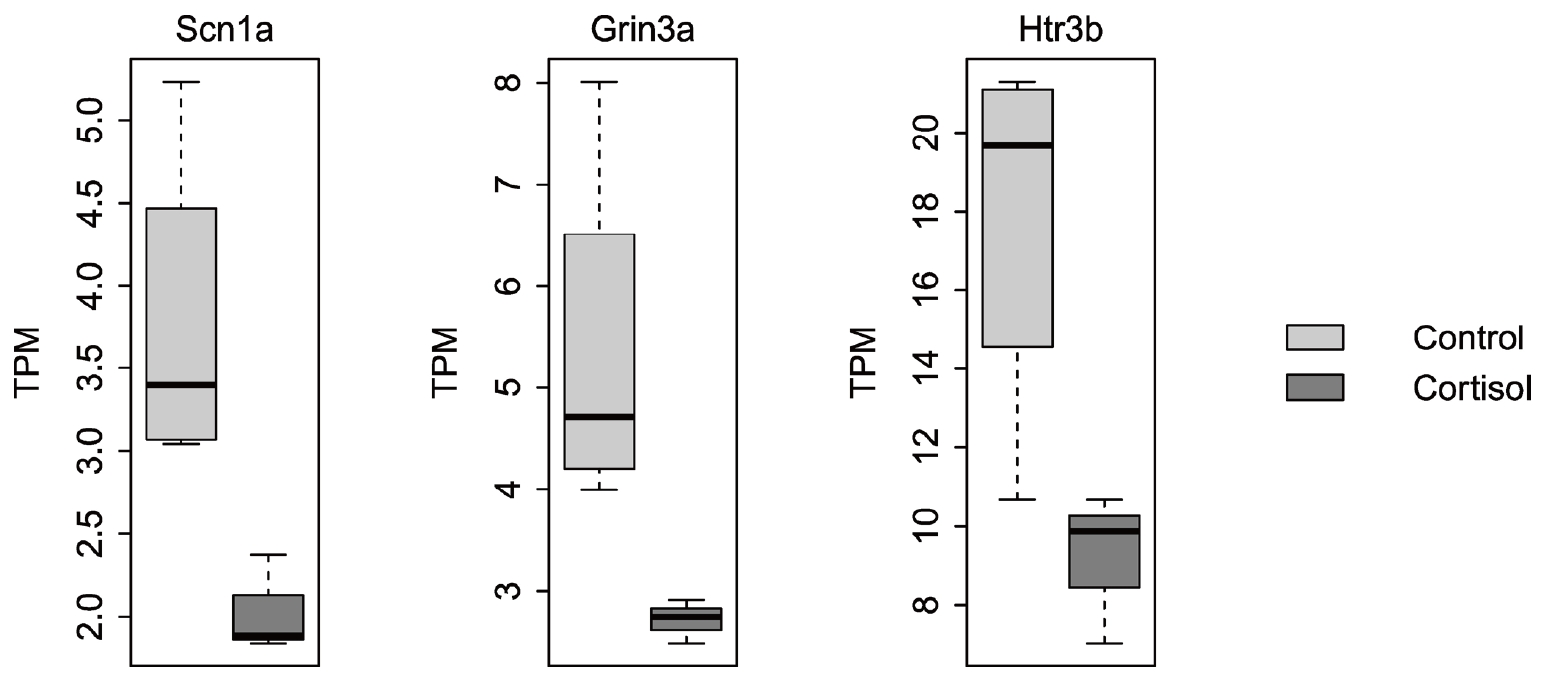
Differentially expressed ion channel genes in pups between controls (n=5) and those born from corticosterone-treated rats (cortisol, n=5). TPM: transcripts per million.
Calcium signaling is an essential component of GI function, as it governs a variable range of signal transduction processes that are involved in smooth muscle contraction, secretion, and sensory signaling. Compared with expression in the control group, pups born to corticosterone-treated rats showed differential expression of various genes involved in regulating calcium signaling. Specifically, the expression of Atp2b2, Hrh1, Nos1, and Ret was reduced, whereas that of Cxcr4 and Tbxa2r was upregulated in pups born to corticosterone-treated rats (Fig. 2). Atp2b2 encodes the plasma membrane calcium ATPase isoform 2, which functions as a calcium pump. HRH1 is a histamine receptor H1 associated with visceral hypersensitivity in irritable bowel syndrome. NO produced by nitric oxide synthase 1 acts as a neurotransmitter in enteric neurons, where it stimulates the release of other neurotransmitters such as acetylcholine and substance P. This release leads to smooth muscle relaxation and increased GI motility, both of which play important roles in the regulation of intracellular calcium levels. Ret proto-oncogene (RET) is a receptor tyrosine kinase that regulates the development of the enteric nervous system and contributes to GI motility and secretion. Impaired RET signaling is associated with various GI disorders, including Hirschsprung’s disease. Congenital megacolon is caused by dysfunction of enteric neurons in the distal colon and severe intestinal dysmotility, with symptoms such as constipation and irritable bowel syndrome. Among the genes related to calcium signaling, higher expression of Cxcr4 and Tbxa2r was identified in pups born to corticosterone-treated rats compared to that in controls. CXCR4 is a chemokine receptor with a fundamental role in the progression of tumor growth and various cancers, including in gastric, colorectal, and pancreatic cancers. Tbxa2r encodes the thromboxane A2 receptor, which is a potent vasoconstrictor and platelet aggregator. TBXA2R dysregulation has been observed in several GI disorders. For instance, upregulated TBXA2R expression has been linked to mucosal inflammation, increased risk of ulceration due to the promotion of gastric secretion, and enhanced tumor cell proliferation in colorectal cancer [4,5].
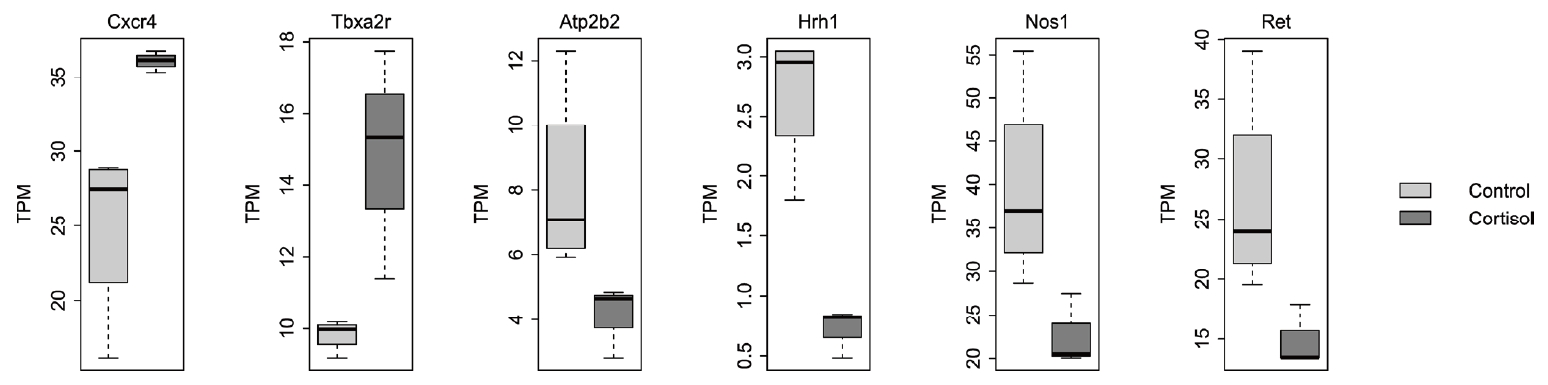
Differentially expressed genes within the calcium signaling pathway in pups between controls and those born from corticosterone-treated rats (cortisol). TPM: transcripts per million.
We investigated ion channels and calcium signaling because of their important impact on GI tract motility. These pathways affect smooth muscle cells, interstitial cells of Cajal (ICCs), and neurons, all of which play crucial roles in GI motility. Therefore, changes in the genes related to ion channels and calcium signaling under stress may affect intestinal motility. Maternal cortisol levels may affect infants not only in terms of cortisol levels but also in their GI functions; for instance, higher maternal stress with elevated plasma cortisol during pregnancy might negatively influence GI functions in infants, leading to issues such as cramping, bloating, inflammation, diarrhea, or constipation. In summary, our results demonstrate that gene signatures related to ion channels and calcium signaling are differentially expressed under high cortisol levels. The motility of the GI tract is controlled by ICC activity. Consequently, RNA sequencing of isolated ICCs is necessary to clarify the dysregulated gene signature contributing to stress-induced GI disorders. Further studies are required to comprehensively understand the importance of each gene in GI disorders.
Supplementary Material
Supplementary Table 1.
Gene name and description
Acknowledgements
EAK was in part supported by the 2023 education, research and student guidance grant funded by Jeju National University.
