Prostate spindle cell neoplasm associated with early voiding difficulty after transurethral resection of the prostate
Article information
Abstract
This report presents the case of 75-year-old men with spindle cell neoplasm. The patient underwent percutaneous nephrolithotomy and transurethral resection of the prostate (TURP) for renal stones and benign prostatic hyperplasia. One month postoperatively, the patient was able to void without any difficulty. Five months later, the patient experienced difficulty voiding and presented to the emergency room with severe pelvic pain. Computed tomography (CT) showed regrowth of the prostate mass into the posterior bladder and penile root. The prostate-specific antigen level remained constant at 1.14 ng/mL during the pre-and postoperative periods. Five months before the TURP operation, the patient’s CT scan showed a soft and mildly enlarged prostate with no protrusion into the bladder. Biopsy of the prostate, however, showed a protruding mass, indicative of a spindle cell neoplasm. The patient was subsequently treated with the chemotherapeutic drug adriamycin. Unfortunately, treatment was unsuccessful, and the patient died 18 months later.
INTRODUCTION
Chronic voiding difficulties in patients with benign prostatic hyperplasia may be attributed to acute urinary retention secondary to outflow obstruction. This outflow obstruction may be a mechanical obstruction caused by physical narrowing of the urethral channel. Another etiological factor for this outf low obstruction may be increased muscle tone within and around the urethra, similar to benign prostatic hypertrophy and hyperplasia [1]. Long-term suboptimal management may also initiate acute urinary retention because of the potential for disease progression [2]. Therefore, surgical management is recommended for decompressing the obstruction of the prostatic urethra. The patients experienced symptom relief after decompression. Even so, voiding difficulties in the immediate postoperative period are common complaints in patients with enlarged prostate. These voiding difficulties may be due to de novo urinary urgency and postoperative infections. Therefore, conservative treatment in some patients during the postoperative period may relieve their symptoms [3]. In the present case, the patient had a considerable amount of disease. The patient reported postoperative improvements in urinary symptoms and stream. However, the urinary symptoms and stream progressively worsened and were not attenuated by conservative treatment. The patient was diagnosed with prostate spindle cell carcinoma. We believe that the neoplasm aggravated the patient’s symptoms after transurethral resection. This case was difficult to manage in the clinic because of the postoperative voiding difficulty. Therefore, this case report may serve as a reference for the management of similar patients. This case was approved by Jeju National University Hospital Institutional Review Board (IRB number: 2021-02-004).
CASE REPORT
A 75-year-old men, who underwent cystostomy in a community hospital due to acute urinary retention (AUR) in September 2018 required treatment for a renal stone in the left pelvis. Consequently, the patient underwent a percutaneous nephrolithotomy in October 2018. The patient was further evaluated for urination disorder. Subsequently, the patient was noted to have an enlarged prostate (70 mL, calculated using transrectal ultrasound) and a prostate-specific antigen (PSA) level of 1.14 ng/mL. Transurethral prostatectomy and cystostomy were performed to treat bladder outlet obstruction in December 2018. The pathologist reported that the benign prostate tissue accounted for approximately 50 mL of the surgical specimen. Postoperatively, the patient was able to void easily. The maximum flow rate (Qmax), voided volume (Vvoid), and residual volume (RV) during the first 2 postoperative weeks were Qmax 13 mL/s, Vvoid 220 mL, and RV 39 mL, respectively. One month later, these values reflected a “good urinary stream” at Qmax 18 mL/s, Vvoid 320 mL, and RV 60 mL. This “good urinary steam” was maintained until March 2019 (postoperative 3 months) when the patient complained of difficulty voiding. The AUR was managed by inserting a 900 mL urinary catheter (900 mL) in the emergency department. Cystoscopic results showed regrowth of the prostate. After a week of resting the bladder and avoiding over-distension with an indwelling urethral catheter, the patient was still unable to void after catheter removal. Therefore, follow-up was performed, self-catheterization was recommended, and the patient was treated with alpha-blockers for 2 weeks. After a week, the patient presented to the emergency department with complaints of discomfort and pain in the perineal region. Digital rectal examination revealed prostate tenderness and overall hardness. Antibiotics and nonsteroidal anti-inflammatory drugs were prescribed for the prostatitis. Four days later, the patient returned to the emergency department and showed worsening pelvic pain. A computed tomography (CT) scan showed regrowth and an enhanced infiltrating mass around the prostate. A transrectal biopsy revealed a spindle cell neoplasm (Figs. 1, 2). The mass metastasized to the lungs and liver and directly invaded the penile root. However, these aggressive masses were not visible on the initial CT scan performed in December 2018 (Fig. 3). Palliative chemotherapy with adriamycin monotherapy was recommended. Primary chemotherapy could not halt disease progression, and the patient died 8 months later.
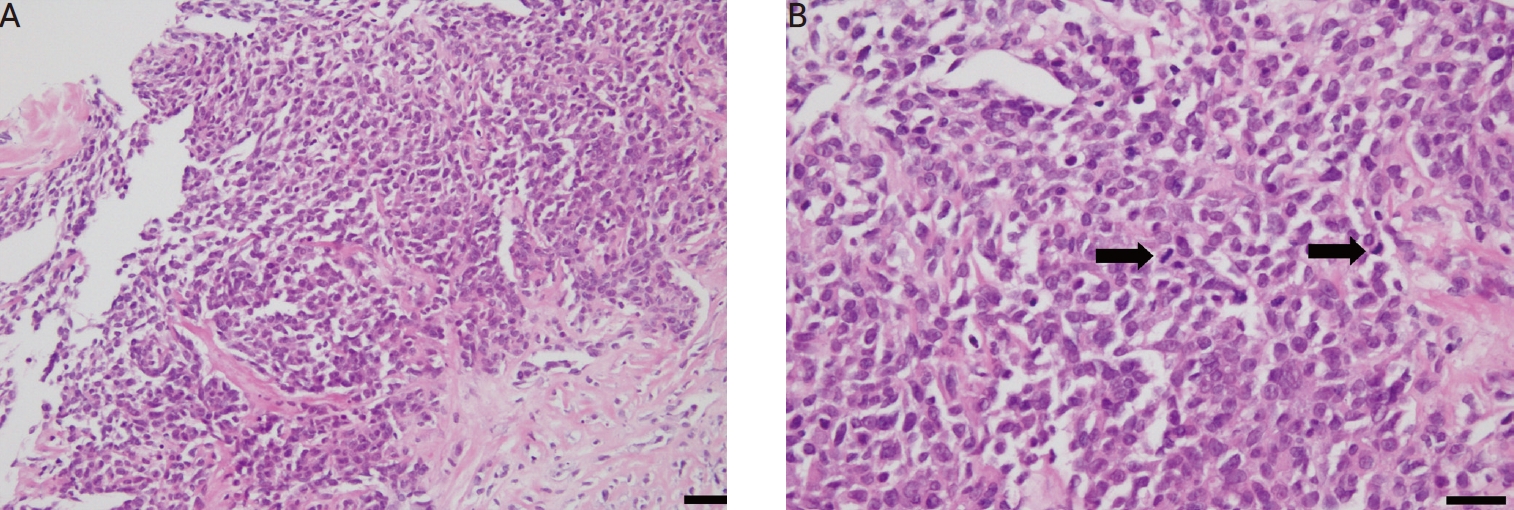
The pathologic features of prostate biopsies. (A) Microscopically, the hematoxylin and eosin (H&E) staining of the needle biopsy specimen revealed perpendicularly oriented fascicles of spindle cells. Monomorphic spindled or oval shaped tumor cells arranged within short intersecting fascicles. The tumor cells had a relatively bright eosinophilic cytoplasm and blunt-end nuclei. Nuclear pleomorphism and atypia were also observed (H&E, ×200; scale bar=100 μm). (B) Frequent mitotic features were observed microscopically (mitotic count: 10/10 high-power fields, H&E. ×400; scale bar=50 μm). Arrows are mitotic feature.
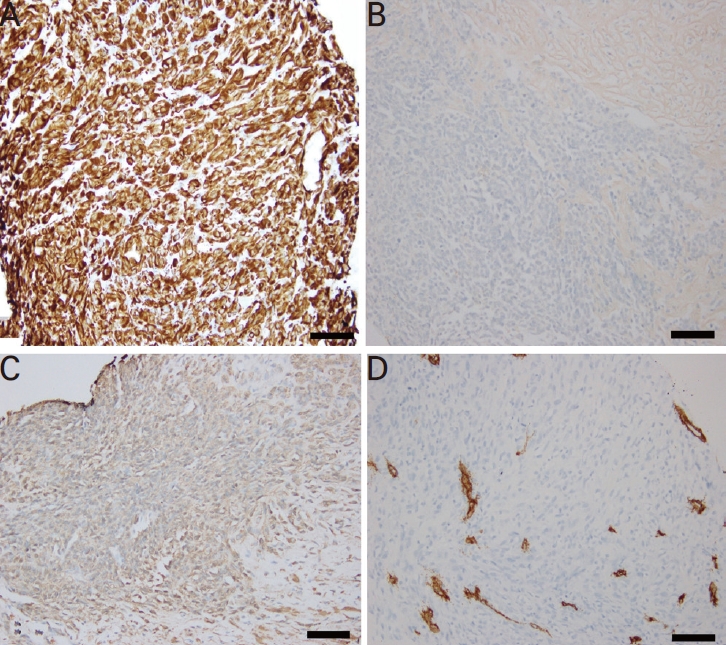
The immunohistochemistry of the biopsy specimens. (A) Tumor cells were strongly positive for vimentin (mesenchymal marker) and (B) negative for cytokeratin (epithelial marker) (immunohistochemistry, ×200; scale bar=100 μm). (C) Tumor cells were weakly positive for smooth muscle actin (scale bar=100 μm) and (D) negative for CD34 (vascular marker), S-100, and desmin (immunohistochemistry, ×200; scale bar=100 μm).
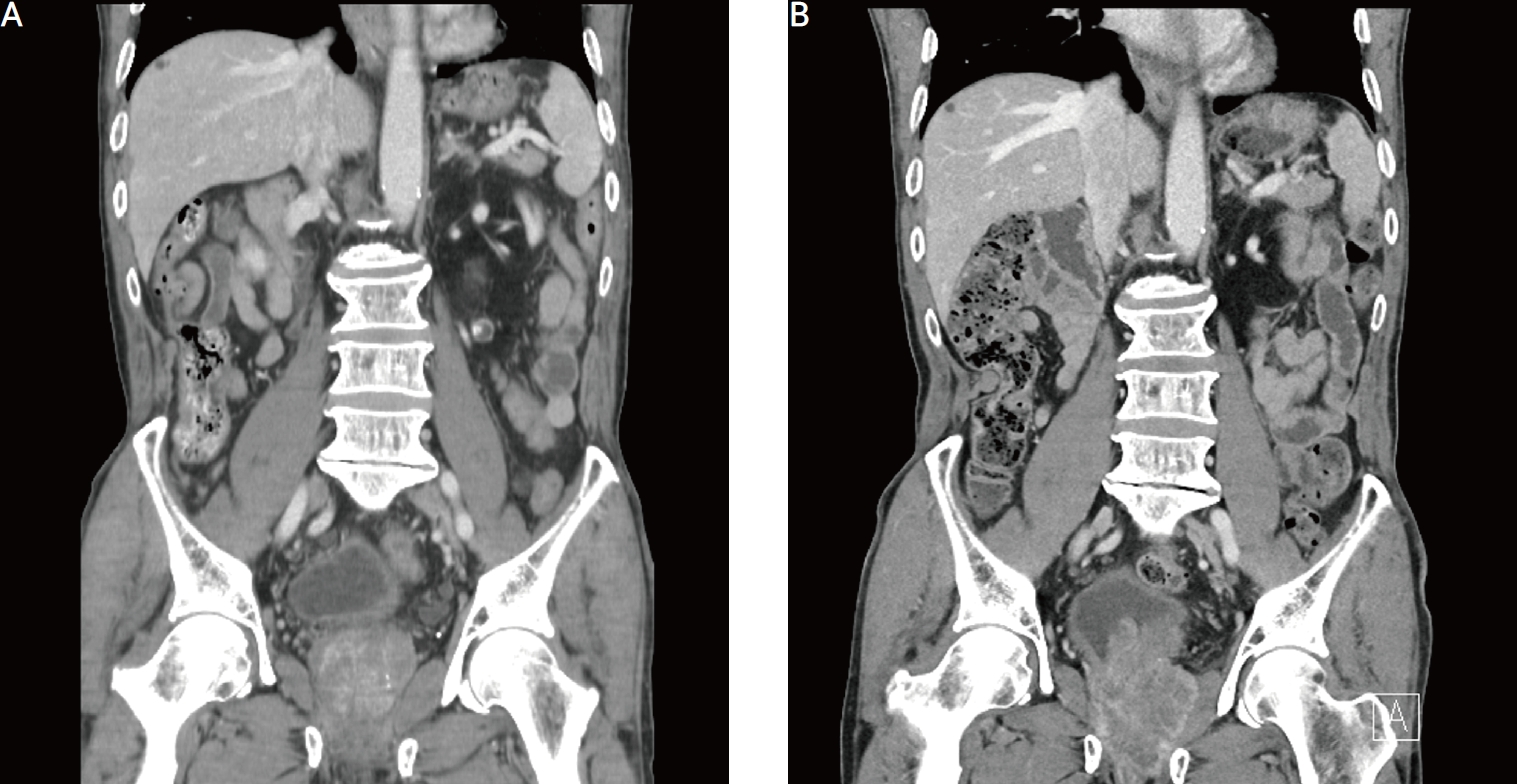
The computed tomographic (CT) image of pre-transurethral resection of the prostate and 6 months before operation. (A) A mildly enlarged prostate was observed in the preoperative CT image. (B) A mass protruding into the bladder was observed in the post-transurethral resection 6 months after the CT imaging.
DISCUSSION
Spindle cell neoplasms are highly aggressive tumors. In the present case, the prostate mass enlarged within 6 months. Imaging and transurethral resection specimens showed benign findings 6 months before spindle cell neoplasm was identified on prostate biopsy. This case demonstrates that spindle cell neoplasms grow rapidly.
We explored a set of hypotheses to identify the mechanisms underlying the observed rapid growth of spindle cell neoplasms. The first etiological factor is the capability of space-occupying cancer cells. Hanahan and Weinberg [4] reported several characteristics of cancer growth. Cancer cells require sufficient blood supply to facilitate cell division. Compacted cells have a limited supply of nutrients and oxygen because of vessel compression. A hyperplastic prostate consists of a high volume of stromal and epithelial hyperplasia [5]. Therefore, transurethral resection of the prostate relieves cellularity and increases the vascular supply to existing cancer cells. This triggers the rapid growth of existing neoplasms within the prostate. Parsons et al. [3] reported that prostate cancer rapidly progressed after palliative transurethral resection of the prostate in men with prostate cancer with neuroendocrine differentiation. The patient’s serum PSA level remained low after resection. However, urethral dilatation for acute urinary retention was required 5 months later, and lower urinary tract symptoms became increasingly evident. The authors concluded that androgen deprivation facilitates tumor progression, resulting in metastasis. Chemotherapy is recommended as an alternative treatment. Although the cell types differed, rapid prostate proliferation was observed and PSA levels remained low after transurethral resection. Wei et al. [6] argued that the suppression of androgen receptors could aggravate the disease. However, we suggest that prostate proliferation should be observed in cases of transurethral resection without male hormone suppression therapy.
Guo et al. [7] reported the prevalence and risk factors for incidental prostate cancer (IPC) in transurethral resection specimens and negative results on prior prostate biopsies. Their results showed that a 4.7% incidence of IPC and risk factors were conventional biopsies rather than fusion prostate biopsy, older age (>70 years), and smaller prostate volume. Karlsson et al. [8] showed that transurethral resection of the prostate may increase the risk of prostate cancer, but decrease prostate cancer mortality in 7,901 benign prostatic hyperplasia cases. The authors said that the increased risk of prostate cancer may be attributed to increased surveillance and PSA screening.
Brockway et al. [9] reported two cases of spindle cell neoplasms in specimens obtained using Holmium Laser enucleation of the prostate. The patients experienced voiding difficulties and severely enlarged prostates. Most of the patients had acute urinary retention. These results are congruent with the findings in the present case. However, the rate of cell transformation in neoplasms is poorly understood. Since imaging sets of pre- and postoperative and pathological results at 6 months intervals, were available, these sets were further evaluated, and they supported the rapid growth hypothesis of the spindle cell neoplasm of the prostate. Farag et al. [10] reported a rapidly growing sarcoma that resulted in mortality after radiotherapy for low-grade prostate neoplasm. In that case, the patient had been diagnosed with Gleason 6 prostate cancer and had undergone radiotherapy on the prostate, his PSA level dropped to 0.62 µg/L, 1 year post radiation. However, he complained of a new onset of voiding difficulty, and his PSA level rose to 2.67 µg/L, 3 years post radiation. Although CT and bone scans showed normal findings, sarcoma of the bladder neck was discovered on cystoscopic biopsy. Finally, the patient underwent pelvic exenteration due to undifferentiated pleomorphic spindle cell sarcoma. Extensive tumor recurrence was noted 1 month later, and the patient was subsequently palliated and died. Choi et al. [11] reported that palliative transurethral resection of the prostate (TURP) as a cytoreductive operation in patients with advanced prostate cancer has limited benefits in the management of patients with prostate neoplasms. However, patients undergoing TURP due to cancer-related symptoms, especially within 3 months of biopsy, had worse clinical outcomes.
In conclusion, rapidly worsening voiding difficulty after transurethral prostate resection affects the growth of existing cancers. Therefore, imaging surveillance of the prostate after transurethral resection is required in patients with rapidly aggravated urine flow.
