Acute aortoiliac thrombosis in minimal change disease
Article information
Abstract
Patients with nephrotic syndrome (NS) are generally known to be at greater risk for thrombosis, with arterial thrombosis-related complications being relatively rare compared to venous thrombosis-related complications. This report describes a 46-year-old male with historically proven minimal change disease (MCD) complicated by acute aortoiliac thrombosis. He had been diagnosed with MCD 8 months previously and was treated successfully with steroids. He was prescribed a second course of high-dose steroids (prednisolone 1 mg/kg/day) due to a relapse of MCD at the outpatient clinic 8 days before the emergency department visit. The patient presented with severe pain in both lower limbs and was diagnosed with aortoiliac thrombosis that developed during high-dose steroid treatment. He subsequently underwent surgical thromboembolectomy. Hypoalbuminemia has the strongest association with the risk of thromboembolism. According to international clinical practice guidelines, anticoagulant therapy is recommended when serum albumin is ≤2-2.5 g/dL. However, as serum albumin levels may be relatively high in the early phase of NS, as in this case report, an individualized anticoagulation strategy for each patient should be considered, regardless of serum albumin levels.
INTRODUCTION
Thromboembolic complications are serious complications in patients with nephrotic syndrome (NS). Venous thromboembolic diseases such as pulmonary thromboembolism, renal vein thrombosis, and deep vein thrombosis are common in patients with NS. In contrast, arterial thromboembolic complications are relatively rare but are associated with more severe consequences [1,2]. A consensus on the initiation of prophylactic anticoagulation therapy in patients with NS has not been established. According to the 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines on glomerular diseases, prophylactic anticoagulation is recommended when the risk of thromboembolism outweighs the potential patient-specific risks of an anticoagulation-induced serious bleeding event [3]. In this paper, we report a case of acute aortoiliac thrombosis that presented when using a steroid to treat a relapse episode of minimal change disease (MCD) without the initiation of prophylactic anticoagulation.
CASE REPORT
A 46-year-old man presented to the emergency department with sudden onset of severe pain, and progressive weakness and numbness in the lower extremities, and back pain that began 6 hours prior to the visit. The patient had a medical history of hypertension and biopsy-proven MCD. At the time of MCD diagnosis, laboratory data were as follows: serum creatinine, 0.76 mg/dL; serum albumin, 2.1 g/dL; total cholesterol, 365 mg/dL; triglyceride, 197 mg/dL; high-density lipoprotein (HDL) cholesterol, 56 mg/dL; and low-density lipoprotein (LDL) cholesterol, 259 ml/dL. The coagulation profile revealed the following: prothrombin time (PT), 12.7 seconds; activated partial thromboplastin time (APTT), 35.8 seconds and international normalized ratio (INR), 0.96. Urinary protein excretion was 7.727 g of creatinine. The laboratory tests to exclude other hypercoagulable disorders did not deviate significantly from the normal range or showed normal findings. Steroid therapy was initiated, and the patient achieved complete response after 4 weeks of treatment, and steroids were tapered for 5 months thereafter. The patient was followed up in complete remission for 8 months, and relapse occurred.
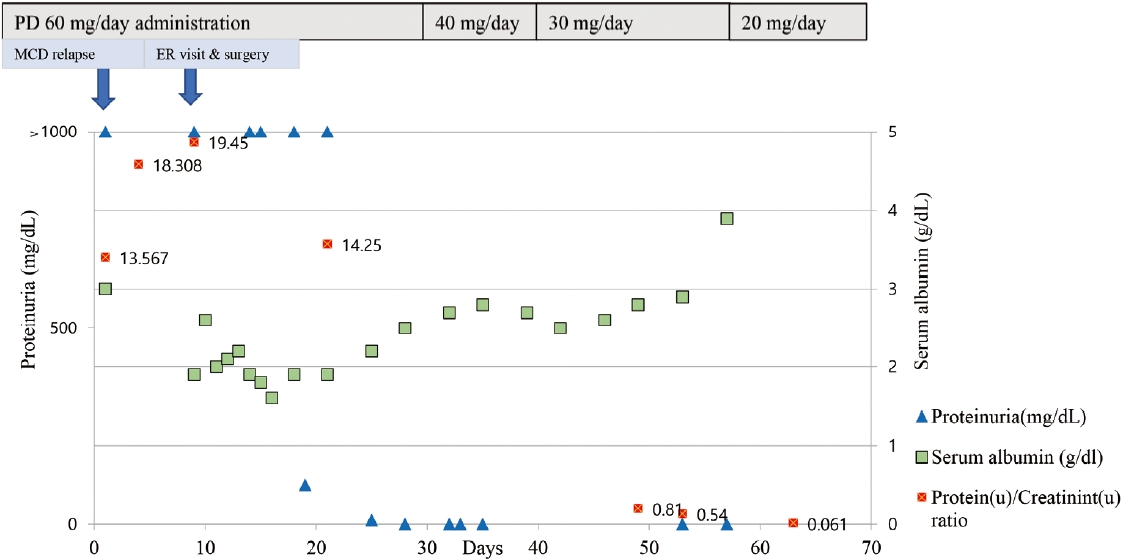
At the outpatient clinic 8 days earlier, urinalysis showed 4+ proteinuria with a protein-to-creatinine ratio of 7.567 g protein/g creatinine (g/g cr) and a serum albumin level of 3.0 g/dL. His lipid profiles at that time were as follows: total cholesterol, 271 mg/dL; triglycerides, 204 mg/dL; HDL cholesterol, 45 mg/dL; and LDL cholesterol, 174 mg/dL, and statins were being taken. MCD relapse was diagnosed, and the patient had been taking prednisolone (1 mg/kg) since then, without anticoagulation. Physical examination findings from the emergency department visit showed that the patient had bilateral cold feet, impalpable bilateral dorsalis pedis, and posterior tibial arteries. Initial laboratory data revealed a white blood cell count of 18,780 cells/μL, a hematocrit level of 52.9%, a platelet count of 240,000 cells/μL, a serum total protein concentration of 4.1 g/dL, a serum albumin concentration of 1.9 g/dL, a blood urea nitrogen level of 24 mg/dL, and a serum creatinine level of 0.60 g/dL. PT and APTT were 12.2 seconds (INR, 0.92) and 35.3 seconds, respectively. Tests to rule out other hypercoagulable disorders including tumor markers (protein C/S activities, antithrombin, factor V Leiden mutation, homocysteine, antiphospholipid immunoglobulin [Ig] G/M, antinuclear antibody, anti-cardiolipin IgG/M, anti-beta 2 glycoprotein I IgG/C, anti-neutrophil cytoplasmic antibody, fibrin degradation products/D-dimer quantitative test, fibrinogen, lupus anticoagulant, and tumor markers including carcinoembryonic antigen, carbohydrate antigen 19-9, prostrate-specific antigen, alpha fetoprotein, and cancer antigen 125) were performed routinely, and the test results did not have any specific findings or were within the normal range. Echocardiography and 24-hour Holter monitor revealed no structural heart disease or arrhythmia. Three-dimensional computed tomography angiography demonstrated total thrombotic occlusion of the abdominal aorta and bilateral common iliac arteries, as well as a contrast filling defect at both the external iliac arteries, left internal iliac artery, and beneath both popliteal arteries (Fig. 1). The thrombus completely blocked abdominal blood flow in the aortoiliac region. The patient underwent emergency surgical arterial thromboembolectomy using a Fogarty balloon catheter, and anticoagulation therapy was initiated. The complete course of medical therapy and serum albumin levels are shown in Figure 2. The patient is currently in remission in terms of NS following the oral administration of steroids. Both lower limbs were salvaged without sequelae.

(A-D) Transverse view of abdominal computed tomography (CT) scan shows aortoiliac thrombosis (arrows). (A) Lower extremity CT shows complete occlusion of the abdominal aorta. CT shows (B) both common iliac artery occlusions, (C) total occlusion of both external and internal iliac arteries, and (D) occlusion of the right external iliac artery, left external iliac artery, and left internal iliac artery while the right external iliac artery remains intact. (E) Coronal view of abdominal CT scan shows total thrombotic occlusion of the aortoiliac region.
DISCUSSION
NS is characterized by hypoalbuminemia, edema, proteinuria, and hyperlipidemia. Due to massive proteinuria, urinary anticoagulant substances are lost, and the liver increases its production of prothrombotic substances, which also leads to a hypercoagulable state. Hypoalbuminemia increases thromboxane A2 synthesis, contributing to platelet adhesiveness. Altogether, these processes result in the hypercoagulable state of NS [4].
Some studies have revealed predictive markers for thromboembolism. Serum albumin levels are a strong predictor of venous thromboembolism in patients with NS because their incidence increases proportionally with lower serum albumin levels [5]. One retrospective study showed that prophylactic anticoagulation in NS with a plasma albumin level ≤2.0 g/dL significantly reduced the incidence of thromboembolic events compared to the untreated group [6].
The Kidney Disease Outcomes Quality Initiative (KDOQI) recommends that prophylactic anticoagulation be considered when the serum albumin level is ≤2-2.5 g/dL and one or more of the following risk factors are present: proteinuria, body mass index, family history of thromboembolism, congestive heart failure, recent abdominal or orthopedic surgery, or prolonged immobilization [7]. The recently revised KDIGO guidelines recommend that prophylactic anticoagulation be employed in patients with NS when the risk of thromboembolism exceeds the estimated patient-specific risk of anticoagulation-related bleeding. Serum albumin levels, used as an indicator of anticoagulation, were the same as those specified by the KDOQI. The albumin level of the patient at the initial outpatient treatment was 3.0 g/dL, which did not meet the prophylactic anticoagulant standard according to the guidelines mentioned above. However, on the day of arrival at the emergency room a few days later, the serum albumin level dropped to 1.9 g/dL. As also seen in this case, serum albumin levels may not be an appropriate indicator of treatment in the early stages of NS as the albumin turnover time is approximately 25 days [8]. Inadvertent application of clinical guidelines to clinical practice, as in our case, may increase the morbidity or mortality of patients due to complications such as thromboembolism. Therefore, individual decisions regarding prophylactic anticoagulation should be preemptively made based on clinical correlations.
One systematic review attempted to determine whether glucocorticoid therapy increased thrombotic risk [9]. The authors concluded that thrombotic diseases are associated with an underlying inflammatory disease that necessitates glucocorticoid treatment, rather than glucocorticoid use. However, a recent study investigated the effect of corticosteroids in healthy individuals, where prednisolone treatment propagated thrombin generation and increased Von Willebrand factor and plasminogen activating inhibitor-1, leading to a procoagulant state in healthy individuals [10]. Therefore, thromboembolic risk is likely to increase during the early stages of NS during highdose steroid therapy. Therefore, if the risk of bleeding is perceived to be low, prophylactic anticoagulation should be initiated in similar cases of MCD.
Prophylactic anticoagulant therapy reduces both morbidity and mortality in patients with NS. Although serum albumin levels are a strong predictive marker for thromboembolism, determining whether to treat anticoagulation based on serum albumin levels alone may put patients at a greater risk. An individualized anticoagulation strategy for each patient should be considered to prevent thromboembolic complications regardless of the serum albumin level. Further research is required to make practical recommendations regarding the initiation of prophylactic anticoagulation therapy.
Acknowledgements
This case was approved by the Institutional Review Board (IRB) of Daejeon Eulji Medical Center (IRB No. EMC 2021-04-007) and the IRB waived informed consent for this reporting.