INTRODUCTION
Urinary tract endometriosis is estimated to affect up to 1% of women with pelvic endometriosis [1]. Bladder endometriosis (BE) is a challenging rare condition that is poorly addressed in terms of pathogenesis, clinical presentation, and management [2,3]. In addition to lower urinary tract symptoms (LUTS), it may lead to obstructive uropathy and renal failure [4]. To achieve the best results, management should involve collaboration between urologists and gynecologists [3]. Herein, we describe a rare case of BE localization at the vesicoureteric junction. Involvement of or proximity to the vesicoureteric junction makes surgical decisions difficult because if the mass is completely resected, the possibility of ureteric damage is high, and if it is conservatively resected, persistence of the disease is possible. In this case report, a female patient diagnosed with BE was properly treated by sequential transurethral resection of BE (TUR BE) and ureteric double J stent ureteric stenting and a postoperative course of dienogest for 3 months.
CASE REPORT
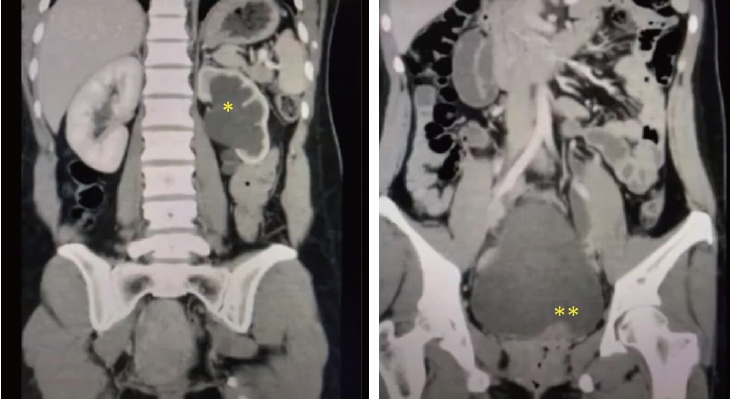
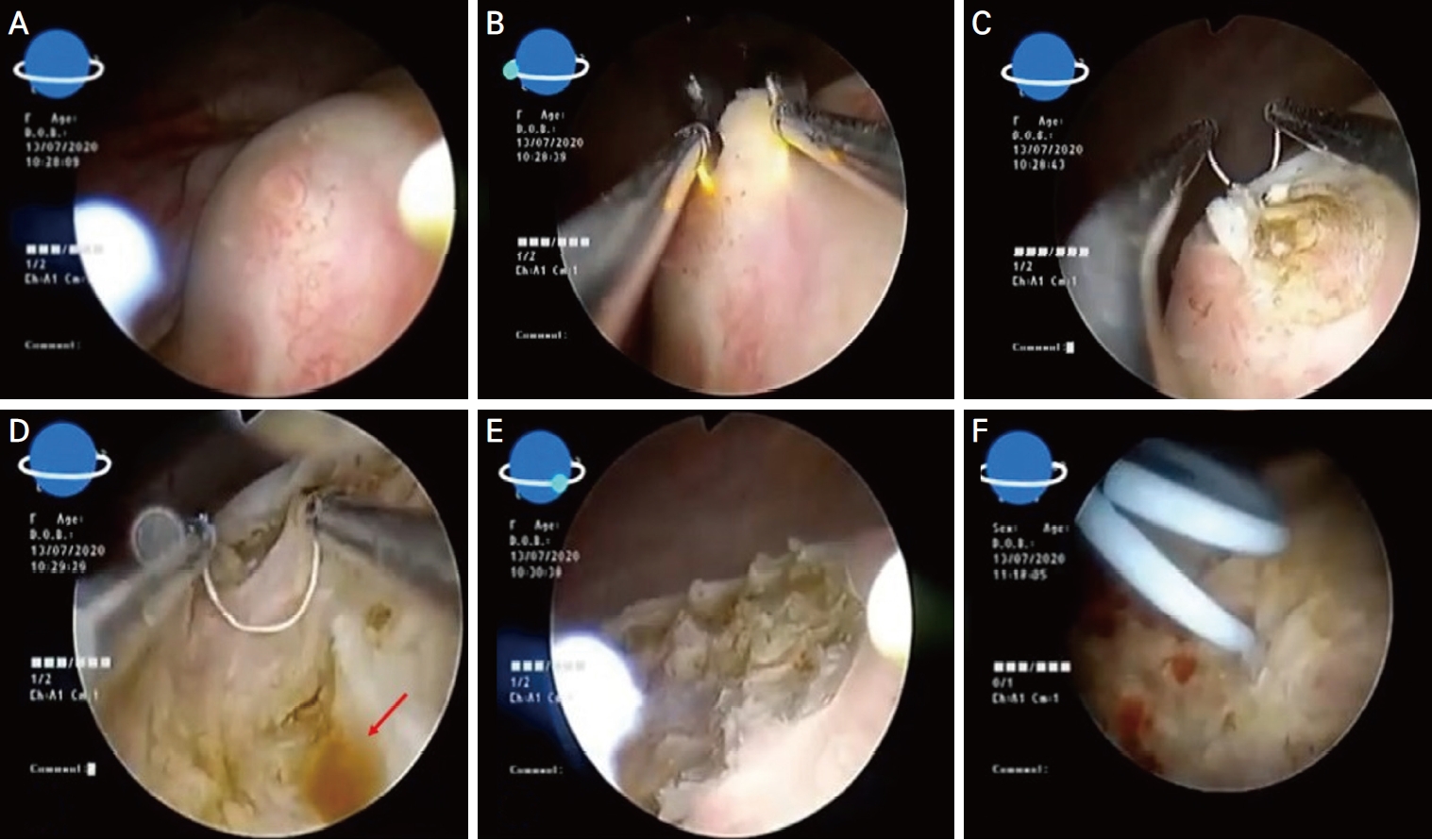
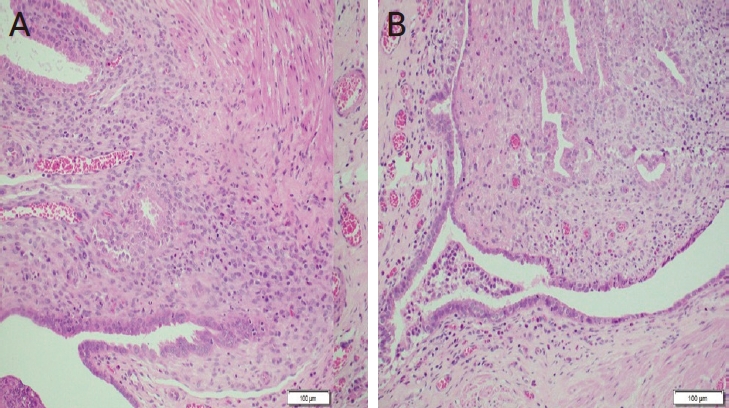
On 7th of July 2020, a 34-year-old multiparous (P4+2) woman presented with dull aching and progressive severe left loin pain for 2 weeks. There was no history of cesarean section, abdominal myomectomy, or other pelvic surgery. She had a history of LUTS in the form of pelvic pain, dysuria, and an increased frequency of micturition characteristically exaggerated just before, during, and persisting for a few days after menstruation. There was no history of hematuria. Clinically, there was left loin tenderness without associated fever. Ultrasonographic assessment revealed marked left renal back pressure, normal bladder wall thickness, and absence of gynecologic abnormalities, apart from a small posterior wall myoma. Multislice Abdominal and pelvic computed tomography (CT) plain, post-IV contrast portal venous and delayed scans showed grade IV left hydronephrosis with a markedly dilated and tortuous left ureter and left soft tissue attenuation lesions at the left vesicoureteral junction measuring 25├Ś15├Ś14 mm, as shown in Figure 1. The patient consented and was prepared for diagnostic/operative cystoscopy and possible concomitant surgical interventions to save the left kidney after receiving approval of the Ethical/Institutional Review Board. Diagnostic cystoscopy revealed a congested mass at the left vesicoureteral junction obscuring the ureteric orifice (Fig. 2A). Complete TUR BE was performed using a bipolar resectoscope (Olympus Medical Systems, Hamburg, Germany) until good exposure of the ureteric orifice was achieved using a DJ stent, as shown in Figure 2B-F. The gynecologist (A.D.) observed chocolate material coming from the mass during resection and highly suspected an endometriotic lesion before the histopathological report (Fig. 2D). As shown in Figure 3, definite endometriotic glands and stroma contributed to the excised mass. The postoperative course was uneventful. An immediate postoperative course of 2 mg dienogest (Visanne; Bayer, Leverkusen, Germany) daily for 3 months was prescribed. On follow-up visits over a period of 2 years, high-resolution 2D ultrasonography revealed restoration of normal renal appearance without any bladder or pelvic masses. Clinically, the patient noted marked improvement of loin pain, attenuated LUTS, and a better quality of life but complained of adverse effects such as headache, breast discomfort, and depressed mood.
DISCUSSION
Endometriosis of the urinary bladder is an uncommon pathology [1,2]. Nevertheless, it should be considered in cases of unexplained dysuria or imaging findings suggestive of urinary bladder malignancy [5]. In this case, transabdominal ultrasonography missed the diagnosis of BE. Even transvaginal ultrasonography may miss the diagnosis of pelvic endometriosis in symptomatic cases [6]. Since transabdominal ultrasonography revealed unilateral hydronephrosis, multislice CT revealed a left markedly dilated and tortuous ureter and a small mass at the vesicoureteric junction. A dedicated preoperative CT or magnetic resonance imaging work-up would help accurately localize BE. Some authors recommend adequate bladder filling during examination to estimate the distance between endometriosis implants and ureteral orifices to better predict the requirement for ureteric stent or resection and reimplantation [7].
Clinicians should consider endometriosis in all cases of pelvic pain or perimenstrual LUTS. Clinical suspicion can be confirmed using endoscopy as the ultimate diagnostic tool. In this case, cystoscopy played a central role in confirming the diagnosis, as well as proper surgical excision. The main problem with the surgical treatment of BE is the proximity of the nodule to the ureteric orifice. Some systematic reviews recommend partial cystectomy or combined surgery to guarantee complete removal of the bladder nodule to minimize recurrence; therefore, transurethral surgery alone should be avoided in favor of segmental bladder resection [1]. This recommendation was not followed in this case report because invasive procedures require laparotomy and ureteric reimplantation with shortand long-term complications such as recurrent urinary tract infections, progressive renal scarring, hypertension, and complications during pregnancy [8]. In contrast, the cystoscopic approach is an ideal minimally invasive procedure with clear and well-settled advantages. It enabled complete resection of the mass with magnified visual demarcation from the healthy bladder tissue. Leakage of chocolate material during resection is an excellent finding, assuring that this lesion is mostly endometriotic rather than malignant. An additional advantage was the use of adjuvant postoperative dienogest to stop the progression of missed endometriotic tissues. This procedure would encourage interested endoscopists to collect similar cases, publish the results, and follow-up for a long time to confirm that there is no recurrence or side effects with the current approach.
Cystoscopically guided resection is key to safe and successful complete excision, as shown in this case. Since there was no gynecological evidence of endometriosis, we omitted the addition of laparoscopy as a diagnostic tool, unlike others [3]. Some case reports have demonstrated the use of the laparoscopic approach for radical excision of BE [5]. We believe that cystoscopic access is easier and more precise, particularly if the mass is suspect in the absence of gynecologic lesions.
In this case report, collaboration between urologists and gynecologists was evident, as gynecologists are more familiar with the diagnosis and management of pelvic endometriosis. The AD observed chocolate material coming from the mass during resection and suspected an endometriotic lesion before the histopathologic report. The gynecologist selected a 2 mg daily dose of dienogest as adjuvant therapy to complete the treatment of the lesion, as recommended by some studies on BE [9]. On follow-up visits performed by urologists, 3-month adjuvant dienogest was considered by the gynecologist to be sufficient to ensure normal bladder, renal, and ureteric anatomy, unlike others who administered it for 12 months [10] The keys to successful BE management in this case were the elimination of symptoms, restoration of normal renal and ureteric anatomy, and absence of recurrence on follow-up visits for up to 2 years. These goals led to the gynecologic decision to stop dienogest after only 3 months. The patient complained of adverse effects such as dienogest-like headache, breast discomfort, and depressed mood, as previously reported [11]. Prolonged dienogest therapy is associated with risks and side effects [12]. Moreover, surgical excision of endometriotic tissue is the ideal treatment for all types of extragenital endometriosis, while adjunctive treatment might be useful in selected cases [4]. It is important to follow treatment guidelines and tailor care to the womanŌĆÖs individual needs and desires [12,13]. Others have used gonadotrophin releasing hormone (GnRH) agonists to treat extragonadal endometriosis [12,14]. Nevertheless, dienogest offers advantages in terms of safety and tolerability [15]. Some studies recommended starting medical treatment before deciding on surgery, with a success rate of 52.3% [16]. In this study, this approach was impossible because the patient presented with advanced renal and ureteric affection that urged surgery as a first-line therapy, while dienogest was added as an adjuvant therapy to ensure the destruction of any remaining endometrial lesions.
The overall recurrence rate of endometriosis ranges from 6% to 67% [17]. On 2-year follow-up, we detected no recurrence in this case, as the mass was properly resected using a resectoscope followed by adjuvant hormonal treatment. In conclusion, BE of the vesicoureteric junction can be properly treated by sequential transurethral resection and hormonal therapy without recurrence over a 2-year follow-up. This rare case report highlights the importance of minimally invasive procedures in modern practice and the value of cooperation between urologists and gynecologists. Urogynecology is an attractive collaborative term used to describe a better management plan for many problems in women. Establishment of urogynecology units in hospitals would improve the healthcare of women and ensure highquality treatment for patients undergoing urogynecological surgery [18].



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print